Add one more to all the amazing things CRISPR can do: it is also the ultimate driving machine. Provided, of course, that you are a gene, and that your destination is any chromosome where you do not currently reside.
Gene Drives Spread by Overwriting Alleles
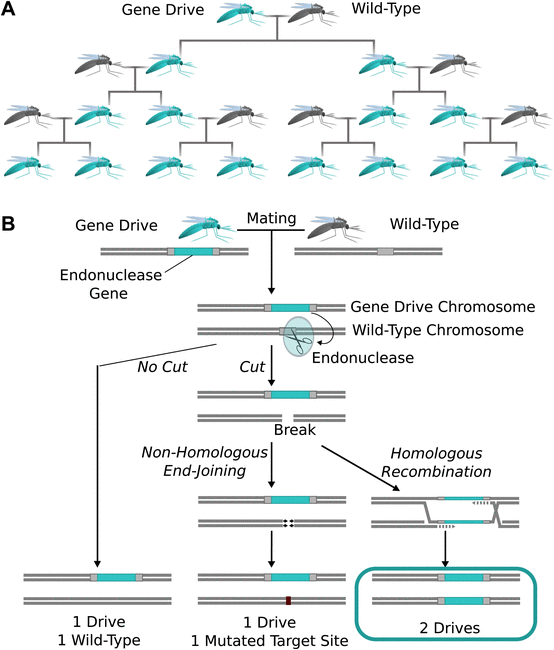
A gene drive is when a gene on one chromosome copies itself onto the other, overwriting the other allele. When a diploid with a gene drive reproduces, no matter how attractive its mate’s genes may be, the gene drive replaces the mate’s allele, and the offspring ends up with two copies of the gene drive version. Over many generations, a gene drive would sweep through a population (Figure 1.). Synthetic gene drives were first suggested by Austin Burt in 2003, but it was CRISPR/Cas9 that made them actually possible.
Figure 1. In a gene drive, all offspring end up inheriting the gene drive allele, which in this case makes them fluorescent turquoise. Reproduced from Esvelt et al. eLife 2014.
Cas9 Makes Synthetic Gene Drives a Reality
In 2014, Kevin M Esvelt, Andrea Smidler, Flaminia Catteruccia, and George Church described how a gene drive with Cas9 at the wheel could cut a target gene and insert itself via homologous recombination. If gene-driven mosquitoes were released into the wild, they could make entire populations malaria-resistant or even control mosquito numbers.
Meanwhile, Valentino Gantz and Ethan Bier were looking for a better way to make homozygous Drosophila mutants. Normally, you’d do that by making heterozygous mutants, breeding them, and looking for the 25% that got both mutant copies. Unfortunately, finding the heterozygous mutants to breed is hard to do for recessive mutations. However, they realized that if a gene mutation included Cas9, it could copy itself over to the other chromosome, and they could easily get homozygous knockouts.
They tested this by making a recessive mutation of a pigment gene called yellow, crossing mutants with wild-type flies, and completely breaking Mendelian inheritance. The number of yellow flies that hatched, way more than standard genetics would predict, “was like the sun rose in the west,” as Bier put it. In trying to make fly genetics easier with what they call a mutagenic chain reaction, they had demonstrated the first synthetic gene drive. It wasn’t 100% efficient; some flies were mosaics (half yellow, half normal), and a few lost the Cas9 insert by not using homologous recombination to repair its DNA cuts, but the second generation was about 97% yellow.
When are Gene Drives a Step Too far?
Not only impressive, the study has also been controversial. In addition to wildly transforming fly genetics, it could also genetically transform wild flies. For that reason, Gantz and Bier kept their flies behind a James Bond-ian series of vials, tubes, boxes, locked doors, fingerprint readers, sharks with laser beams on their heads, and other BSL-2 security. That wasn’t enough for heavyweight George Church though, who called publishing this work “a step too far” and is promoting an alternate gene drive method. While this paper was in press, Church’s group posted a preprint of their method in yeast, with Cas9 supplied externally on a plasmid, rather than being inserted as part of the new allele. That way, if the gene-driven yeast did escape, they would eventually lose the plasmid and the gene drive would hit a wall, avoiding safety concerns. All well and good for yeast, but as Bier points out, the plasmid method can’t work in flies.
Buckle up; the road ahead for gene drives may be bumpy, but it’s going to be one hell of a ride.
Make your own homozygous flies with Science, March 2015.