If you don’t run chromatin immunoprecipitation (ChIP) experiments on a daily basis, there’s a good chance that you find the dizzying variety of available immunoprecipitation bead choices a little overwhelming. We asked the ChIP experts from EMD Millipore to help us break down what you need to know about ChIP beads so that you choose the best set up to use in your lab.
Magnetic vs. Agarose ChIP Beads
The first step is to figure out which of the two main types of beads you want to start with, ones with magnetic cores or the more traditional agarose-based kind. Here are the major differences between them:
Magnetic Beads
Advantages
- Low non-specific binding
- No blocking and pre-clearing required for most magnetic beads
- Magnetic separation makes them easy to handle during washes
- Beads are visible in tube
- Highly reproducible results
Disadvantages
- Slightly higher cost
- Magnetic rack required (typically lower cost than the microfuge used for agarose beads)
- Non-porous, which leads to lower binding capacity, but lower backgrounds
Agarose Beads
Advantages
- High capacity binding. Porous structure increases surface area.
- Simple common laboratory equipment required (centrifugation or filtration)
- Slightly lower cost
Disadvantages
- Blocking and pre-clearing required
- Increased non-specific binding possible due to porous nature
- Not visible in tubes
- High probability of bead loss during handling leading to less consistent results
So, magnetic beads provide rapid isolation of protein/DNA complexes from crude chromatin mixtures; while on the other hand, agarose beads are a less expensive, high-capacity binding option that uses less specialized equipment. Both varieties have their pros and cons, but it seems like most researchers these days are drawn to the magnetic beads. John Rosenfeld, PhD, Epigenetics R&D Group Leader at EMD Millipore explains why. “In direct comparisons at EMD Millipore, we have seen little difference in performance, measured by signal or S/N ratio, in the assays we’ve run in between agarose and magnetic beads, so we prefer magnetic because of the ease of handling. They are frankly easier to use in washing and result in a cleaner separation of bead from sample.”
Protein A, G or A/G Beads: What’s the Difference?
ChIP antibody:chromatin complexes can be captured by either agarose or magnetic beads which come in a variety of flavors, most commonly protein A, protein G, or in the case of EMD Millipore a blend of protein A and G conjugates (protein A/G).
The protein A beads have the best affinity for rabbit polyclonal antibodies, while protein G beads bind a wider range of antibodies including most mouse monoclonal IgGs. The protein A/G blends allows for the most antibody flexibility since it combines the binding characteristics of both protein A and protein G; essentially giving you the best of both worlds.
“We’ve noticed that we often get a reduction in ChIP background when Protein A and G beads are blended.” Commented Rosenfeld. That seemingly simple switch to A/G blended beads can make a huge difference when it’s time to analyze the data. According to Rosenfeld, “It varies depending on the antibody, but in general, we get better positive signals and reduced background when testing blended versus unblended beads in the dozens of antibodies we’ve evaluated. This results in a greater fold enrichment, or signal to noise ratio when performing ChIP experiments.” Here’s just one example of the improved performance EMD Millipore has seen by offering antibodies a blend of protein A and G capture molecules.
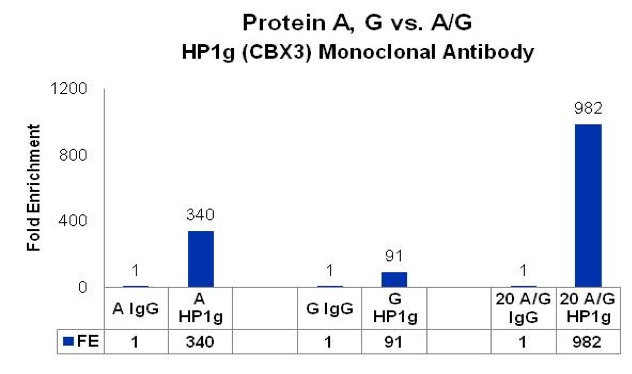
qPCR analysis of chromatin immunoprecipitated with protein-G purified mouse monoclonal HP1g or IgG and isolated with protein A, G, and A/G magnetic beads. HP1g- and IgG-associated DNA were immunoprecipitated from HeLa cells as described in the user manual for the ChIPAb+ HP1g antibody primer set (cat# 17-646). Immunocomplexes were collected using 20 uL protein A, G, or A/G magnetic beads and the Magna GrIP magnetic separation stand (cat# 20-400)
In this case the A/G blend delivered a whopping 982-fold enrichment compared to protein A (340) or G (91) alone. As EMD Millipore’s Rosenfeld puts it, “We’ve come to the conclusion that blending beads can mitigate the fact that binding preference is not always predictable.“
Make Your A/G ChIP Beads a Premium Blend
You can find protein A/G beads that we’ve discussed here available on the EMD Millipore website:
- Magna ChIP™ Protein A+G Magnetic Beads
- EZ-Magna ChIP™ A/G Chromatin Immunoprecipitation Kit
- Magna ChIP™ A/G Chromatin Immunoprecipitation Kit
- Magna ChIP-seq™ Chromatin Immunoprecipitation and NGS Library Preparation Kit
To see EMD Millipore’s complete offering of kits, antibodies and reagents for ChIP click here