Antibody vendors have been hard at work to offer antibodies that are suitable for applications like ChIP. Usually these antibodies have been used successfully in peer-reviewed publications. In many cases they’ve been validated internally by an R&D team, and in some cases, each lot may be validated to work in ChIP applications.
The tricky part is that vendor terminology isn’t uniform, so ChIP “Grade,” “Qualified,” or “Validated”, may mean different things depending on the vendor. Pay close attention to the QC description that your vendor provides. If it’s not possible to tell from website information, get on the phone with technical service to clarify. Here are some explanations of the ChIP validation naming mechanisms from a few of the largest ChIP antibody manufactures, EMD Millipore, Diagenode, and Abcam.
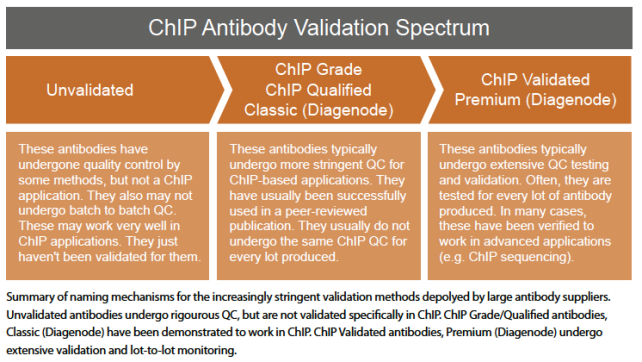
ChIP Grade & Qualified
The ChIP “Grade” or “Qualified” tag usually denotes that an antibody has been used successfully in real-life ChIP experiments. This level of validation is definitely a useful indicator of the quality, but it’s important to remember that antibodies, (especially polyclonals) can exhibit variation in performance over time, even when they’re drawn from the same host animal, so you still might want to validate its performance to be certain. Here’s a little more detail on exactly what this level of validation means at three large ChIP antibody suppliers.
At Abcam, ChIP grade means the antibody has been extensively tested and used successfully by researchers in their own experiments (a ‘real-life’ ChIP experiment). They cite published scientific papers & customer reviews (Abreviews®) on each of the antibody datasheets to ensure all data on the products is easily available to their customers. If, after reading the product datasheets, you have further questions you can always speak to the Scientific Support team who works with Abcam’s Epigenetics specialists and laboratory to answer any other queries.
EMD Millipore doesn’t use the term ChIP Grade. Instead, they refer to antibodies that have been used successfully in a publication or by collaborator for ChIP, ChIP-chip or ChIPseq as ChIP Qualified. In all cases the published reference and/or collaborator data is included in the certificate of analysis as well as the EMD Millipore website.
Diagenode uses ChIP-grade to describe those antibodies that have undergone extensive QC testing and validation and that have been verified in certain applications such as qPCR. However, Diagenode now uses a different categorization system for its antibodies that focuses on “ChIP-seq grade” antibody classification for many of their antibodies, given the sensitivity and popularity of ChIP-seq. Diagenode has a three-tier antibody classification system in which antibodies are categorized by their degree of validation. The antibody classes include “Premium,” “Classic,” and “Pioneer.”
The “Premium” antibodies receive their status after undergoing thorough and rigorous validation by actual epigenetics experts in various large epigenetics consortia. These antibodies have passed stringent bioinformatics tests from those defined by the NIH ENCODE project criteria and the Broad Institute reference data matching for ChIP-seq. Diagenode performs all antibody validations inhouse for ChIP and ChIP-seq antibodies.
The “Classic” antibodies include those that have been validated and cited by epigenetics researchers in a number of peer-reviewed citations for use primarily in ChIP or ChIP-seq.
ChIP Validated
Usually, this moniker is tagged onto more thoroughly tested ChIP antibodies. We say usually because, like any other ChIP naming mechanism, there can be exceptions with certain companies. EMD Millipore uses “Validated” for their highest level of QC in their ChIPAb+ Antibody and primer sets. “With ChIPAb+ antibodies, you’re not only getting an antibody that’s been verified to work in ChIP, but you also get a set of negative IgG and positive locus primers that you can use as controls in your own experiments,” shared Michael Sturges, PhD, Sr. Product Manager at EMD Millipore.
He continued, “For an antibody to be considered validated and earn ChIPAb+ status it must pass a very rigorous screening process as well as lot-specific QC testing. First, multiple antibodies are chosen as candidate ChIPAb+ antibodies, and screened for crossreactivity, background signal, and their ability to enrich at a known positive locus.
Additionally, the antibodies are evaluated for background signal at genomic locations where they are not expected to enrich. “Antibodies that show the best biologically significant enrichment have their ChIP conditions further optimized. The antibody that consistently shows the best enrichment is then put forward as a ChIPAb+. However, that’s not the end of the process. Each and every lot of a ChIPAb+ is checked in ChIP experiments to ensure that every vial of a ChIPAb+ antibody performs reliably in ChIP.”
“A large selection of Abcam’s ChIP-grade antibodies receive our highest possible level of validation. This means that every single batch of these antibodies is initially tested for specificity and cross-reactivity,” Davide Mantiero, PhD, Abcam
Abcam’s “ChIP Validated” antibodies exist within their ChIP Grade offering. Davide Mantiero explains “A large selection of Abcam’s ChIP-grade antibodies receive our highest possible level of validation. This means that every single batch of these antibodies is initially tested for specificity and cross-reactivity. We then extensively test the antibody in ChIP at as many genomic loci as possible to really make sure that we only stock the batches that we know will perform the best in researchers’ experiments.” Diagenode’s “Premium” line of antibodies are about as validated as antibodies get. With an emphasis on ChIP-Seq, Diagenode runs their antibodies through an obstacle course of QC checks and balances, but we’ll talk more about them in our ChIP-Seq Grade Antibodies section below.
Application-Specific Validation: ChIP-Seq Grade Antibodies
ChIP sequencing has transformed the utility of ChIP by providing unprecedented genome resolution and coverage. But the associated costs, time, and data management involved in ChIPseq make having a validated antibody more important than ever. In general, a ChIP Grade, ChIP Qualified, or ChIP Validated classification in itself does not guarantee the antibody has been actually used in a ChIP sequencing setting.
Sometimes you’ll have to dig a bit deeper into the specific validation method provided, or the publications that a vendor lists for that particular antibody.
All three of these suppliers make it easy to see if an antibody has been used in a ChIP-seq setting. For Abcam, just check out the “Tested Applications” section on the datasheet, or review the “Specific references” for the antibody. For EMD Millipore, lists of all proven applications are included on their certificates on analysis provided with every antibody. To find these antibodies simply search for “chip-seq” on www.emdmillipore.com/antibodies.
Diagenode has a section dedicated to their ChIP-seq validated antibodies as well.
“In the last 3 years, ChIP-seq has become the most sensitive and accurate approach for the whole genome study of epigenetic marks such as histone modifications or transcription factor binding sites. This is why Diagenode has invested most of its efforts in establishing standardized methods for ChIP-seq antibody validation.” Dominique Poncelet, PhD, head of Diagenode R&D
Poncelet shared a little more insight into Diagenode’s characterization and quality control workflow for their ChIP-seq grade antibodies, which are primarily directed against the most relevant epigenetics targets such as modified histones or transcription factors:
- Following immunizations, rabbit crude serum is tested for immune response.
- Next, the antibody’s specificity is determined by using complementary methods such as Western blot, immunofluorescence, peptide arrays, and peptide competition assays, especially important for histone modifications.
- Then the most specific antibodies are tested in ChIP using an optimized method for antibody screening.
- After the ChIP QC, the antibodies are evaluated in a ChIP-seq experiment, using one of two different type of NGS machines (Ion Torrent and Illumina sequencers)
- They then evaluate the antibody in a standardized ChIP-seq analysis method based on the ENCODE and modENCODE recommendations and proprietary bioinformatics software settings including peak calling and rigorous QC methods.
“Therefore each validated antibody that passes our strict quality control can be supplied to researchers in a reproducible manner, eliminating the requirement for validation by the customer,” added Poncelet. Dominique Poncelet, PhD, head of Diagenode R&D
