Gene drives have been hitting the news quite a bit lately, generating a lot of hype, hope, and some hand-wringing. They are a relatively new concept, so it’s well worth a quick road trip into gene drive history, domestication, safety, and practical challenges.
Gene Drives?
In essence, gene drives are the wormholes of biology. They look like they’re breaking science, but they’re actually just sidestepping around the rules on a technicality. There is, however, a key difference between wormholes and gene drives: wormholes have a Matthew McConaughay movie, whereas gene drives have fruit flies held behind locked, fingerprint-reading doors.
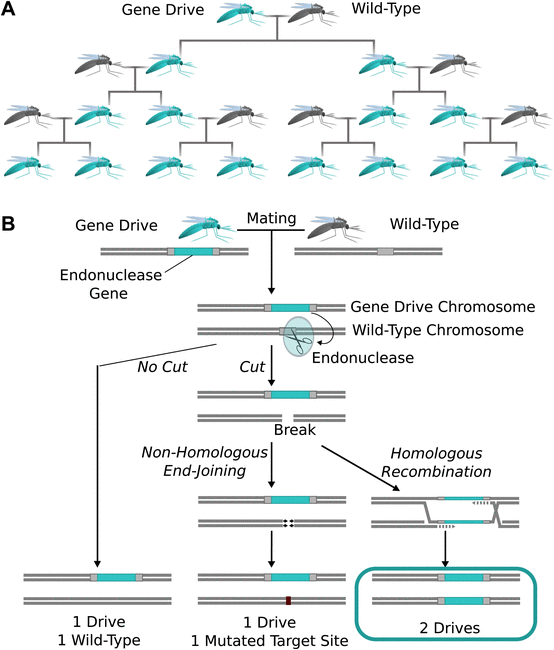
In a gene drive, the drive allele is (almost) always passed on to offspring, instead of the 50% inheritance predicted by Mendelian genetics.
Like wormholes, gene drives are super-fast ways to get where you’re going, they just warp evolution instead of spacetime. The concept behind a gene drive is deceptively simple; it just means that a gene is passed down to offspring – and, therefore, spreads through a population – more than standard genetics would predict. If we could piggy-back some useful trait onto a gene drive, we could manage entire populations on the genetic level, and much faster than classical selection.
Making that idea actually work has taken decades, but we have finally seen a few practical examples. This harnessing of gene drives gives us an incredibly powerful new tool, one which could potentially wipe out scourges like malaria or protect crops without toxins.
Gene Drives in the Wild
Gene drives aren’t a human invention; several types exist out in the wild. For example, selfish genes can destroy matching chromosomes that don’t carry themselves during meiosis, “Medea elements” can prenatally kill competing siblings, and transposons can copy themselves into other regions of the genome. These selfish genes would be very difficult to harness for a controllable gene drive, but there is one more type: site-specific selfish genes.
Site-specific selfish genes insert themselves into DNA, replacing particular spots in the genome. Then they sit there and watch for the DNA sequence they replaced. If their cell ever merges with another cell containing that DNA, i.e., via sex, they cut that target site. The host cell then searches frantically for a homologous sequence to repair its broken DNA with, finds the chromosome with the selfish gene on it, and uses that as a template for homology-directed repair (HDR). If all this happens upon rendezvous between sperm and egg, the organism that grows out of it will have the selfish gene on two chromosomes instead of just one, and as the process repeats with the circle of life, the selfish gene spreads exponentially down the generations.
Domesticating Gene Drives
In 2003, Austin Burt suggested that site-specific homing endonucleases – genes that cut specific DNA sequences – could be harnessed to create a useful gene drive. Not too long after, Burt’s lab, along with those of Andrea Crisanti and Steven Russell, demonstrated this by inserting endonuclease cut sites into fruit fly or mosquito genomes and driving the matching endonuclease into those target sites. Of course, this would only work in pre-engineered genomes, so it demonstrates the key bottleneck for gene drives – the endonuclease has to recognize the target site.
As Austin Burt said in his original proposal, it would only be really useful given “the ability to engineer [selfish] genes to recognize a new target sequence.” With this goal, Alekos Simoni tried to use engineered Zinc Finger and TALE Nucleases to make gene drives in Andrea Crisanti’s lab, but these nucleases are hard to work with, and they picked up mutations after just a few generations because they’re so repetitive.
However, if you’ve been following synthetic biology, you’ll realize that “the ability to… recognize a new target sequence” sounds a lot like CRISPR. Indeed, 2014 saw a “hey, heads up” paper on CRISPR-guided gene drives (from Kevin Esvelt, Andrea Smidler, Flaminia Catteruccia, and George Church), and right on its heels, 2015 saw the first few demonstrations.
CRISPR Gene Drives
Interestingly, the first published CRISPR-mediated gene drive wasn’t even really meant to be a gene drive. Valentino Gantz was a graduate student of Ethan Bier at UC San Diego, studying fruit fly wing development. To answer his wing vein questions, he needed to make some homozygous flies (i.e., with the same mutation on both chromosomes). Unfortunately, making homozygous flies is long and painstaking. The initial selection only mutates one chromosome, so the process takes several generations of flies and lots of tedious screening.
As an end-stage graduate student, Valentino did not have time for all this. However, he realized that if only the initial mutation could copy itself onto the other chromosome, he could get one-step homozygous flies and finally graduate. Of course, Valentino and Ethan soon realized that this Mutagenic Chain Reaction (MCR) – replacing a gene with cas9 and a gRNA targeting the replaced gene – is also exactly what you’d need to make a gene drive. Indeed, not only did Valentino easily make homozygous flies using this MCR, but when he bred them with wild-type flies, nearly all the offspring were homozygous for the mutation – an impossible result according to standard Mendelian genetics!
Of course, the labs of George Church and Kevin Esvelt, having issued the 2014 “heads up”, were working on gene drives at the same time. Not long after, James DiCarlo and Alejandro Chavez demonstrated a series of gene drives in budding yeast. Meanwhile, two groups demonstrated gene drives in mosquitos, hoping to make good on the promise of malaria control.

This guy, with the malaria it carries, is one of the world’s major killers. Just imagine if we could ecologically manage it not with sprays, but with genes.
In one approach, Valentino Gantz and Ethan Bier teamed up with Nijole Jasinskiene in Anthony James’ lab to drive anti-malarial genes into mosquitoes. These antibodies should prevent mosquitoes from carrying the parasite. In an alternative approach, Andrew Hammond, hailing from the labs that had been pioneering non-CRISPR drives, made a suppression drive to knock down the actual mosquito population.
Suppression drives, which were modeled by Anne Deredec, would drive down populations by sterilizing one sex or biasing sex ratios. Andrew Hammond made several such drives knocking out different female fertility genes in germline cells. In theory, only the drive allele should be passed on by heterozygous bugs, spreading it through the population. As it spreads, more and more of the females would be homozygous, which would render them sterile and hold down the population.
Gene Drive Safety
Humans have always changed other species, from domestication and breeding to inadvertently selecting for smaller-horned sheep by trophy hunting. Gene drives, however, could alter populations much faster and much more precisely, so it’s crucial to consider how to control them and make them safe.
There are a few reassuring natural features of gene drives. First, drives only pass from parent to offspring, so they’re not contagious. This also means they would take too long to be useful in long-lived animals, like humans. Also, they’re not a concern in bacteria or viruses that don’t reproduce through sex, which is how gene drives spread. And finally, we can easily detect them with ever-cheaper DNA sequencing.
It’s still wise to take precautions when working with gene drives, and the labs that created these first drives proposed a set of standard operating procedures:
- Physical Containment: Test plants and animals should be kept in secure labs, with multiple barriers against escape.
- Ecological Containment: Experiments should use organisms that can’t reproduce with wild populations, even if they do escape. This could mean using a special strain or using species outside their natural geographic range.
- Molecular Containment: For experiments not intended for use in the wild, the cas9 gene and gRNA should be separated. For example, cas9 could be pre-integrated elsewhere in the genome or expressed on an episome in a special experimental population. Then a gene drive containing only the gRNA would not be able to spread through wild populations.
Even if gene drives did escape or become a problem, it still wouldn’t necessarily be a problem. We could also control drives with the fighting-fire-with-fire approach, as suggested in the 2014 heads-up paper. Reversal drives could target and eliminate changes made by an original drive, and immunization drives could prevent an unwanted drive from spreading by recoding its target site. In fact, the yeast paper headed by James DiCarlo demonstrated drive reversal, although it did leave behind a residual genomic “scar”.
What’s The Catch?
Ok, so gene drives sound simple enough, but what about the little tricks every experiment needs to actually make it work? For example:
How efficiently do gene drives actually spread?
Oh, you want actual numbers, you scientist? Fine, the drive inheritance rates have all been around 98% in yeast, flies, and mosquitos, at least for the first few generations.
Does the size of the drive matter?
Apparently not, at least not as far as we’ve tried. 5 kb drives spread in yeast as efficiently as 500 bp. In mosquitoes, a 17 kb drive worked fine.
Does the molecular containment strategy screw up the drive?
No, or not in yeast, at least. Expressing Cas9 from a plasmid outside the actual gene drive worked just as well as an autonomous drive containing both cas9 and the sgRNA.
What about non-homologous end-joining (NHEJ)?
Ah yes, that nemesis of CRISPR gene editing: NHEJ. This could be a big problem for gene drive spread because it often changes the target site just enough to prevent the drive from recognizing and cutting it. This would create a new resistant allele that the gene drive couldn’t spread through.
In insects, at least, NHEJ is definitely a problem. The fly MCR, which expressed Cas9 in all cell types, made flies that nearly all looked homozygous for the target gene knockout. However, closer PCR inspection showed most flies were actually mosaics, with some cells in each fly having been repaired by HDR and others by NHEJ.
The Gantz et al. anti-malarial mosquito drive looked over more generations, and they found NHEJ problems by the fourth generation. This drive expressed Cas9 only in recombination-proficient germline cells, which should either replace or destroy the corresponding wild-type chromosome during meiosis. When the drive parent was female, however, the eggs presumably still had some Cas9, which cut the sperm allele and caused drive-breaking NHEJ before the germ line had a chance to differentiate. The Hammond et al. mosquito suppression drive also saw some NHEJ, but by five generations it still didn’t have much effect on the drive’s spread. The yeast drive didn’t look beyond three generations, so it’s not clear if NHEJ would become a problem.
Premature DNA cutting in fertilized eggs might cause more NHEJ because the sperm DNA got cut before it was paired with the egg chromosomes, meaning the template for HDR was just too far away for the drive allele to get copied over. In addition to advances to CRISPR tech, another potential fix could be only expressing the drive in the male germ line.
Epigenietorial
Researchers have known about natural gene drives for a while and theorized about using them for almost as long. With the advent of CRISPR, they can finally harness them and time warp Mendelian genetics. If the community can figure out the NHEJ problem, this could open up a whole new world of ecosystem management, with malaria control as just one possibility.
With great power, however, comes great responsibility, and the labs at the forefront of gene drives have laid out a set of safety measures to prevent accidental spread in the wild. Even if something does backfire, we can prevent or reverse gene drives, and it’s important to remember gene drives can’t be caught and can’t spread to anyone but offspring. Gene drives are quite shiny new vehicles; it will be interesting to see where this road goes!
Further Reading:
- Burt, A. Site-specific selfish genes as tools for the control and genetic engineering of natural populations. Proceedings of the Royal Society of London B: Biological Sciences 270, 921–928 (2003). DOI: 10.1098/rspb.2002.2319
- Chan, Y.-S., Naujoks, D. A., Huen, D. S. & Russell, S. Insect population control by homing endonuclease-based gene drive: an evaluation in Drosophila melanogaster. Genetics 188, 33–44 (2011). 10.1534/genetics.111.127506
- Windbichler, N. et al. A synthetic homing endonuclease-based gene drive system in the human malaria mosquito. Nature 473, 212–215 (2011). 10.1038/nature09937
- Simoni, A. et al. Development of synthetic selfish elements based on modular nucleases in Drosophila melanogaster. Nucleic Acids Res 42, 7461–7472 (2014). 10.1093/nar/gku387
- Esvelt, K. M., Smidler, A. L., Catteruccia, F., Church, G. M. & Tautz, D. Concerning RNA-guided gene drives for the alteration of wild populations. Elife 3, e03401 (2014). 10.7554/eLife.03401
- Gantz, V. M. & Bier, E. The mutagenic chain reaction: a method for converting heterozygous to homozygous mutations. Science 348, 442–444 (2015). 10.1126/science.aaa5945
- DiCarlo, J. E., Chavez, A., Dietz, S. L., Esvelt, K. M. & Church, G. M. Safeguarding CRISPR-Cas9 gene drives in yeast. Nature Biotechnology (2015). 10.1038/nbt.3412
- Gantz, V. M. et al. Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi. Proc Natl Acad Sci 112, E6736–43 (2015). 10.1073/pnas.1521077112
- Hammond, A. et al. A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nature Biotechnology (2015). doi:10.1038/nbt.3439
- Deredec, A., Burt, A. & Godfray, H. C. J. The population genetics of using homing endonuclease genes in vector and pest management. Genetics 179, 2013–2026 (2008). 10.1534/genetics.108.089037
- Akbari, O. S. et al. Safeguarding gene drive experiments in the laboratory. Science 349, 927–929 (2015). 10.1126/science.aac7932



