CRISPR/Cas9 may be the flashiest gene editing tool out there right now, but there are certainly other contenders for that throne. We previously profiled Argonautes as one alternative; another is peptide nucleic acids, or PNAs. Like DNA, PNAs are strings of standard nucleotide bases, but instead of a charged phosphate backbone, they are held together by uncharged, peptide-like bonds.
When a PNA finds a complementary DNA sequence, it invades the double helix and grabs the target strand by the Watson-Crick H-bonds (when you’re a PNA, they just let you do it). The PNA then wraps around and forms Hoogstein pairs with the other side of the target strand, forming a PNA-DNA-PNA triplex. This triplex calls in highly efficient DNA repair machinery, which can efficiently edit DNA if supplied with a donor template for homologous recombination.
Delivering PNAs with polymer nanoparticles, Raman Bahal and Nicole McNeer, working at Yale under Peter Glazer, tackled β-thalassemia – a genetic hemoglobin disease. While making a strong case for PNA nanoparticles, the team also made one interesting discovery that could help gene editing by any technique.
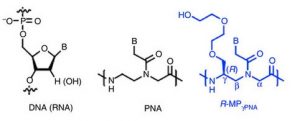
Monomers of DNA, PNA, and γPNA, where B is a nucleotide base. Bahal et al. 2016
SCF: A General Strategy To Increase Editing?
Experimenting with mouse bone marrow stem cells, Bahan and McNeer first tested adding mini-PEG groups at the γ-position of some or all PNA units, since this had previously been reported to help DNA strand invasion (compare the center and right structures above). Indeed, partial γ substitution almost doubled gene editing to 1.6%. Interestingly, editing was much higher for CD117+ cells, at about 8%. When the team added SCF, which is the ligand for c-Kit, the protein product of CD117, gene editing about doubled. Conversely, inhibitors of the c-Kit pathway cut editing efficiency back to that of non-CD117+ cells.
Wondering why CD117 would increase editing efficiency, the team deep sequenced gene expression in CD117 + and – cells, finding upregulation of several DNA repair genes. Expression of these genes increased even further with SCF, suggesting it as a generic strategy for improving gene editing.
Effective Gene Therapy for β-thalassemia In Vivo
Using their two optimizations – partial γ substitution of the PNAs and treatment with SCF – the team was able to hit 15% gene editing for mouse bone marrow cells and 5% for human cells in vitro. When they tried injecting nanoparticles into β-thalassemic mice, they saw an improvement in blood hemoglobin that lasted at least 140 days.
While PNAs still lag a bit behind CRISPR in gene editing efficiency, they have the safety advantage of not being a nuclease, like Cas9. Indeed, double-stranded DNA breaks were nearly undetectable upon PNA treatment, and off-target editing of close sequence matches remained extremely low.
If you want to grab some DNA by the Watson-Crick H-bonds, don’t do that. But if you want to read more on using PNAs for gene editing, check out Nature Communications, 2016.